

Per Teodor Cleve and independently by Marc Delafontaine and Louis Soret Jöns Jacob Berzelius and Wilhelm Hisinger Joseph Priestley and independently by Carl Wilhelm ScheeleĪntoine-Jérôme Balard and Carl Löwig in Heidelberg

Louis-Josef Gay-Lussac and Louis-Jacques Thénard and Humphry Davy Sir William Ramsay and independently by Per Teodor Cleve and Nils Abraham Langlet You Gotta Know These Chemical Elements Hydrogen (atomic symbol H, atomic number 1) is the first element on the periodic table and, by far, the most common. The list of 118 chemical elements with name, atomic number, symbol, atomic weight, electronic configuration, and discovery is given below the table, List of chemical elements The general electronic configuration of inner transition chemical elements are (n-2)f 1 to 14(n-1)d 0 or 1 or 2ns 2. These three incomplete shells are outer ns orbital, (n-1)d orbital, and (n-2)f orbital. The atoms of these chemical elements have three incomplete outer shells.
#Chemical element a series
They formed two series such as lanthanoids and actinoids. Therefore, f block elements are also called inner transition chemical elements. Essentially, this means elements are like different building blocks used to construct matter. The inner transition elements are those elements whose 4f and 5f orbitals are progressively filled by electrons. A chemical element is a form of matter that cant be broken into smaller pieces by any chemical reaction. Atoms of these elements have (n-1)d 1 to 10ns o or 1 or 2 general electronic configurations. They are placed between s- and p-block chemical elements. The outermost two shells in transition elements are incomplete. These chemical elements lie on the extreme right of the periodic table. Non-metals, metalloids, and inert gases are included in these categories. The outer electronic configuration of the atoms of p-block elements varies from ns 2np 1 to ns 2np 6. They are extremely electropositive in nature. The members of s-block elements lie on the extreme left of the periodic table.Īlkali metals and alkaline earth metals are included in this class. The outermost electronic configuration of s-block chemical elements varies from ns 1 to ns 2. The representative or normal chemical elements contain some metals, all non-metals, and metalloids. They have very little tendency to form chemical compounds due to the highly stable ns 2np 6 configuration or valence shell configuration (except He 1s 2). The atoms of these chemical elements have completely filled s or p-subshells. The lack of chemical reactivity of noble gases was the reason to call them inert.
/GettyImages-82020791-58b5b5193df78cdcd8b1cb22.jpg)
All these tables are arranged in the increasing order of their atomic numbers.įour types of chemical elements are found on the periodic table depending on the nature of the atomic orbitals in which the last electrons enter. The post-Mendeleev developments and in order to remove the defects of the Mendeleev periodic table a number of tables have been suggested for the classification of chemical elements. Mendeleev in 1867 first time attempted to classify chemical elements according to their atomic weight. Elements in the periodic table participate in different types of chemical bonding such as ionic bonding, covalent bonding, or metallic bonding to form substances. For example, most noble gases have names ending with -on, while most halogens have names ending with -ine.The periodic table classification of these 118 chemical elements (metals, non-metals, metalloids) is based on the modern periodic law.
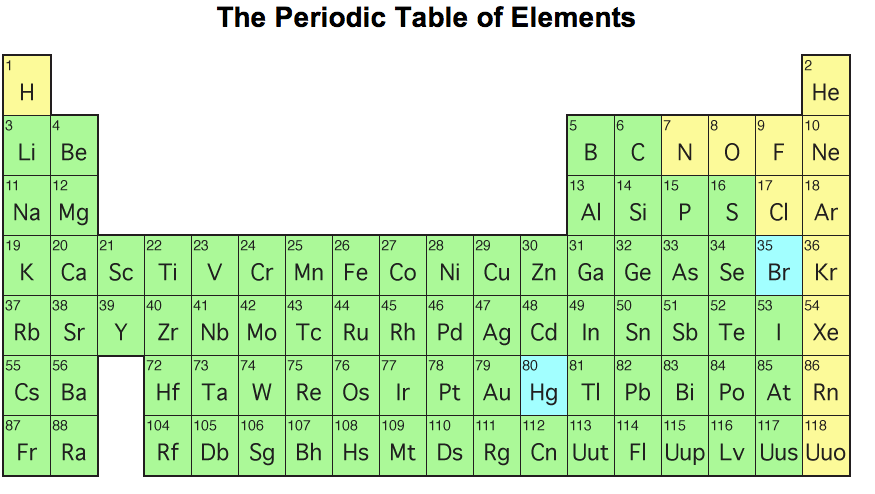
Each element has a symbol, which is one or two letters.The periodic table lists the elements in order of increasing atomic number.Each element is identified by the number of protons in its atoms.There are 118 elements on the periodic table.


 0 kommentar(er)
0 kommentar(er)
